Make an interlocking crystal hearts necklace! Fun science & craft activity for kids.

Have you tried making crystals yet? They’re so pretty! This is our fourth ‘crystal craft’ we’ve made so far. We’ve also made crystal flowers, crystal candy canes, and a (preschooler-friendly) crystal snowy tree. This is our first interlocking-hearts design though!
Suitable for
Making Borax crystals is a classic science experiment for school aged kids. I’d recommend it for 5-6 year olds and up. (Jewel was almost 6 years old here). Older kids could experiment with a few different designs and materials, and see which works best.

How to make a Borax crystal hearts necklace
We used:
- Borax (which you can buy at the grocery story in the laundry aisle, or online here)
- pipe cleaners (also called chenille stems)
- ribbon or fishing line (optional)
- scissors
- boiling water
- spoons
- glass jars
- pencils
- safety glasses (we have these ones)
To make our crystal hearts, the first step is to create the interlocking hearts shape. Cut a pipe cleaner in half. Bring the two ends of one half together and twist, making a loop. Thread the other half through the loop, and then bring those two ends together and twist making a second loop that goes through the first one. Pinch each loops to make two interlocked heart shapes.

We made two sets of interlocking hearts – one for Jewel and one for me.
We decided to make ours into necklaces, so I tied a long piece of ribbon onto the end of mine. Jewel opted for blue pipe cleaners for her necklace. She twisting two pipe cleaners together to make an extra long one, and then twisting the ends of this around her hearts. (If you didn’t want to turn your hearts into a necklace, then I’d tie on something thin and slippery, like fishing line or floss, which the crystals won’t stick to and you can cut off later.)
The next step is to tie the ribbon / fishing wire / pipe cleaners to a pencil or chopstick, so the hearts can dangle below when your pencil is sitting on the rim of your glass jar. Measure it and check. (Don’t skip this step!) You need to make sure that your hearts dangle inside the glass jar, without touching the bottom or the sides. Make sure there’s a little bit of extra room too, as your hearts will become hard and slightly larger when they have crystals on them, and you want to make sure they don’t get stuck inside the jar. If in doubt, choose a larger container, or squish your hearts to make them smaller.
If you have safety glasses, put them on. It’s not strictly essential, but being safety concious around chemicals is a good habit. My girls love wearing their safety glasses whenever they get a chance – it makes it feel like a ‘real’ science activity! (If you don’t have any safety glasses, just make sure they don’t put any Borax in their eyes.)
With an adults help, fill your glass jars with boiling water. Add several tablespoons of Borax and stir until all the Borax is suspended (and looks dissolved). The actual quantity of Borax will depend on the volume of your jar – allow for about 3 tablespoons per 250ml of water.
(Please see safety notes at the bottom. Borax is not taste-safe. If you’ve happened to touch any of the Borax powder, please wash your hands. )

Lower the hearts into the solution so that the pencil is sitting on top of the jar. Double check that your hearts aren’t touching the bottom or sides. Then place your jars in a safe spot where they won’t be disturbed.
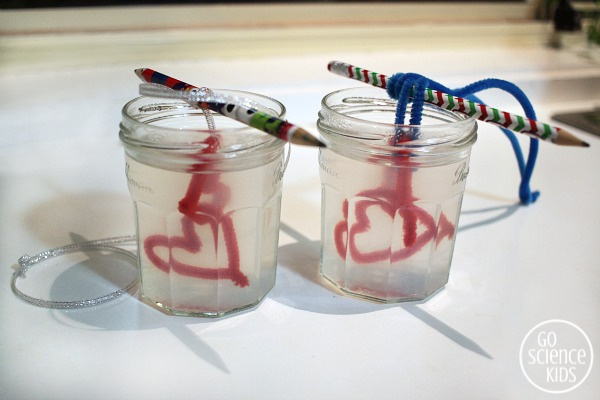
Over the next 24 hours, your crystals will grow! You should be able to see some of the progress through the sides of the glass jar. It’s fascinating! The longer you leave them, the bigger they’ll grow, up to a point. We usually try to leave ours a few days.

Once the crystals have stopped growing (or your patience has run out), remove them from the solution and pat dry. You can rinse them off in fresh water if you want to. Untie your ribbon / pipe cleaner / fishing line, and admire your new crystal hearts!

There’s likely to be some crystals that have formed on the inside of the jar – these are pretty too!

Fun Science Fact
Borax (also known as sodium borate, sodium tetraborate or disodium tetraborate) is a naturally occurring mineral, that is mined from seasonal lakes. You don’t see it in crystal collections in museums though, because if it is allowed to fully dehydrate, it loses it’s structure and eventually crumbles into a white powder. Coincidentally it’s also, strangely enough, used as a detergent alternative and laundry booster, which is why you’ll likely be able to find it in your grocery store’s laundry aisle.
Water is made up of tiny molecules. If you mix Borax with water, some Borax can become ‘suspended’, which means that the water molecules can ‘hold’ onto it for a while. When water is very hot, the water molecules move further apart, and make room for the water molecules to ‘hold’ onto more of the Borax. As the water cools, the water molecules come closer together, and the water molecules can’t ‘hold’ the same quantity of Borax as they could before. This is called supersaturation. The extra Borax separates from the water molecules, and form crystals which stick to the pipe cleaners (and to the bottom of the jar).

The crystals formed are a mineral. They are hard. They are faceted. You can easily see their shape with your naked eye, but they are even more fascinating under a magnifying glass or a microscope.


Your hearts will be ‘stuck’ together. If you’re game, you could try to break them apart, so the two crystal hearts are loosely entwined again. Some of the crystals may break off in this process though!


Here’s Jewel, wearing her necklace. Doesn’t she look chuffed with her efforts!

Please note…
Be very careful with boiling water around young kids. Glass jars with boiling water inside will become too hot to touch.
Borax is a commonly used natural ingredient in grade school science experiments, and is safe for older kids to handle when used responsibly. However it is not edible, will irritate if put into eyes and is a mild skin irritant for people with sensitive skin.
With Borax being inedible, please make sure that your beautiful crystal hearts are stored out of reach of babies or toddlers.
All kids’ activities on this blog require attentive adult supervision. Parents and carers will need to judge whether a particular activity is appropriate their child’s age and skill level. Click here for more information.

For more heart-themed science craft ideas, you might also like our DIY heart-shaped bubble wands. (What shape bubbles will they make??)
And for more crystal ideas, you might also like:
We also have a pinterest board dedicated to growing crystals and crystal crafts!
And you can always subscribe to our newsletter, to receive all our latest posts via email. We’d love to have you join our growing email community!
* This post contains affiliate link(s). An affiliate link means I may earn a referral fee or commission if you make a purchase through my link, without any extra cost to you. It helps to keep this little project afloat. Thank you so much for your support.

#
#